The Evolution of GLP-1 Agonists: From Diabetes Treatment to Cardiovascular Protection
December 4, 2025
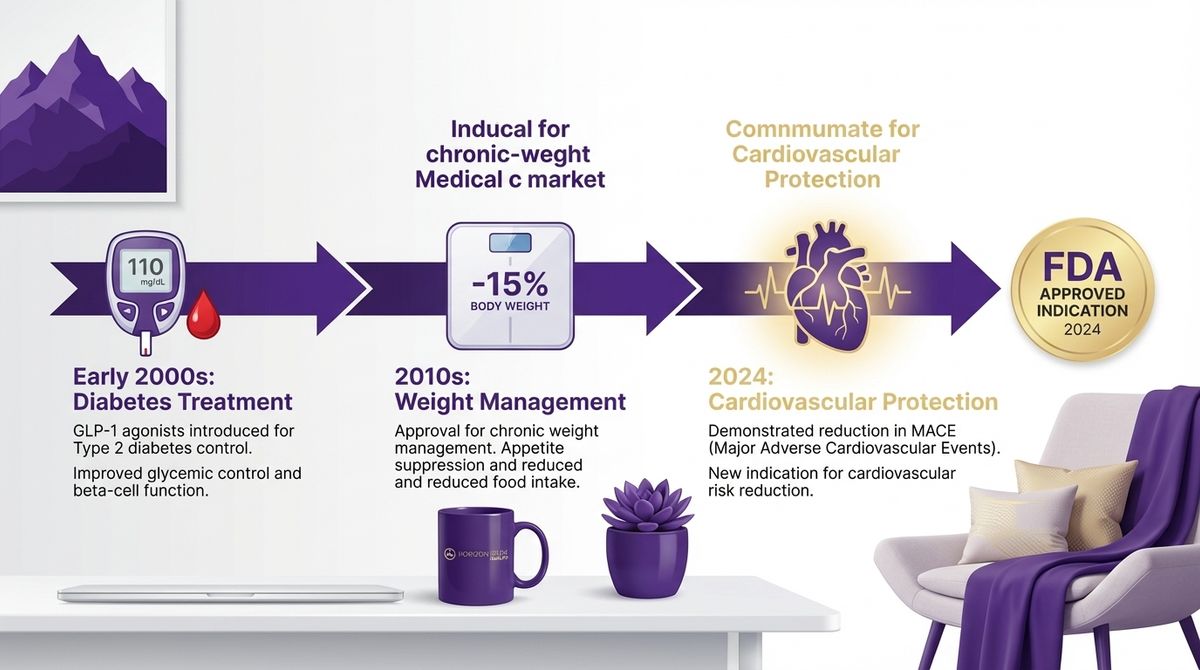
The landscape of metabolic health and cardiovascular disease prevention has been dramatically transformed by the emergence of GLP-1 receptor agonists. Recent developments, including a groundbreaking FDA approval and new comparative effectiveness data, are reshaping our understanding of these medications' potential (Ferhatbegovic et al., 2023).
Recent FDA Milestone
On March 8, 2024, the FDA made a historic decision by approving Wegovy (semaglutide) for cardiovascular risk reduction in adults with cardiovascular disease who have obesity or overweight. This approval was based on a large-scale clinical trial involving 17,600 participants, where Wegovy demonstrated significant reduction in major adverse cardiovascular events (MACE) - 6.5% in the treatment group compared to 8% in the placebo group (FDA, 2024).
Understanding GLP-1 Receptor Mechanisms
GLP-1 receptors are widely distributed throughout the body, including in cardiomyocytes and blood vessels. These receptors play crucial roles in:
Glucose regulation
Cardiovascular function
Blood pressure control
Endothelial function
Inflammatory response modulation(Ferhatbegovic et al., 2023)
Comparative Effectiveness: A New Frontier
Recent research from Baylor University Medical Center has provided compelling evidence regarding the comparative effectiveness of different GLP-1 based therapies. In a large-scale study of 72,424 patients with diabetes, researchers compared cardiovascular outcomes between tirzepatide and semaglutide (Cervantes et al., 2024).
Key Findings
The study revealed significant differences in cardiovascular outcomes:
1. Acute Myocardial Infarction:
Relative Risk: 1.44 (95% CI: 1.10-1.88, p=0.0072)
131 events with semaglutide vs. 91 with tirzepatide
2. Stroke:
Relative Risk: 1.69 (95% CI: 1.27-2.25, p=0.0002)
127 events with semaglutide vs. 75 with tirzepatide
3. Mortality:
Relative Risk: 1.33 (95% CI: 1.05-1.67, p=0.016)
166 deaths with semaglutide vs. 125 with tirzepatide
4. Composite MACE:
Relative Risk: 1.51 (95% CI: 1.29-1.76, p<0.001)
401 total events with semaglutide vs. 266 with tirzepatide
Clinical Implications
These findings suggest that dual agonism (GLP-1 and GIP) with tirzepatide may offer superior cardiovascular protection compared to selective GLP-1 receptor agonism with semaglutide. This has significant implications for clinical decision-making, particularly in patients with high cardiovascular risk (Cervantes et al., 2024).
Future Directions
While these results are promising, additional research is needed to:
Understand the mechanisms behind the differential effects
Identify specific patient populations who might benefit most
Evaluate long-term outcomes
Assess cost-effectiveness implications
The Bottom Line
The FDA's March 2024 approval of Wegovy for cardiovascular protection marks a turning point in metabolic medicine. Key takeaways:
- Wegovy demonstrated significant MACE reduction: 6.5% vs 8% in the placebo group across 17,600 participants
- Tirzepatide may offer superior cardiovascular protection: Relative risk reductions of 33-69% compared to semaglutide in key outcomes
- Dual agonism (GLP-1 + GIP) shows promise: The mechanism behind tirzepatide's potential advantage warrants further investigation
These developments suggest we need to reconsider current treatment algorithms, particularly when cardiovascular risk reduction is a primary goal alongside weight management.
---
Considering GLP-1 Therapy?
If you're exploring GLP-1 agonists for weight management or metabolic health, a comprehensive evaluation ensures you're a good candidate and helps identify the right medication for your specific situation.
What to expect:
- Thorough medical history and cardiovascular risk assessment
- Lab workup including metabolic panel and relevant markers
- Discussion of medication options, including cost considerations
- Monitoring plan for side effects and efficacy
Investment: Initial evaluation and follow-ups covered by most insurance plans (cash rates: $350-450 initial, $150-200 follow-ups; sliding scale available for uninsured).
Locations: Psychiatric evaluation in Rancho Palos Verdes, Psychiatric evaluation in Phoenix, Psychiatric evaluation in Chandler, and telehealth throughout California and Arizona
---
Disclaimer: This article is for educational purposes only and does not constitute medical advice. GLP-1 agonists are prescription medications with specific indications, contraindications, and potential side effects. Treatment decisions should be made in consultation with a qualified healthcare provider who can evaluate your individual cardiovascular risk factors, medical history, and treatment goals. This content does not replace professional medical evaluation.
---
References
Cervantes, M., Miles, B., & Mehta, A. (2024). Comparison of cardiovascular outcomes in patients with diabetes treated with tirzepatide versus semaglutide: a multi-institutional analysis. European Heart Journal, 45(Supplement 1), ehae666.2907.
FDA. (2024, March 8). FDA Approves First Treatment to Reduce Risk of Serious Heart Problems Specifically in Adults with Obesity or Overweight. U.S. Food and Drug Administration.
Ferhatbegovic, L., Mrsic, D., & Macic-Dzankovic, A. (2023). The benefits of GLP1 receptors in cardiovascular diseases. Frontiers in Clinical Diabetes and Healthcare, 4, 1293926.
Canybec Sulayman, PMHNP-BC
Diagnostic Psychiatry Specialist
Investigating the root causes of mental health symptoms with 19 years of ICU diagnostic rigor.